Zunveyl (benzgalantamine) for Alzheimer’s disease
Last updated Aug. 1, 2024, by Marisa Wexler, MS

What is Zunveyl for Alzheimer’s?
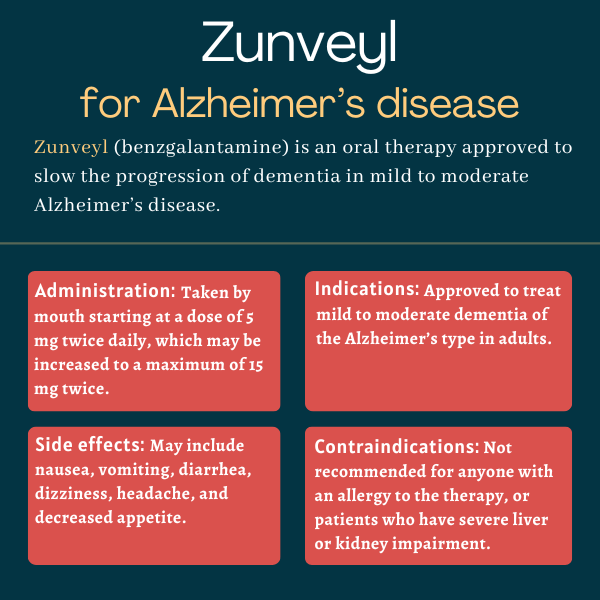
Zunveyl (benzgalantamine) is an approved oral therapy used to slow the progression of dementia in people with mild to moderate Alzheimer’s disease.
Developed by Alpha Cognition, Zunveyl is designed to increase the levels of acetylcholine, an essential neurotransmitter involved in learning, thinking, and memory processes.
Available as delayed-release oral tablets, Zunveyl delivers its active substance only after it passes through the digestive system, thereby potentially improving its tolerability profile.
Zunveyl is also being investigated in combination with memantine for moderate to severe Alzheimer’s dementia, and as an intranasal formulation for individuals experiencing cognitive impairment associated with mild traumatic brain injury.
Therapy snapshot
| Brand name: | Zunveyl |
| Chemical name: | Benzgalantamine |
| Usage: | Used to slow the progression of dementia in patients with mild to moderate Alzheimer’s |
| Administration: | Delayed-release oral tablets |
How does Zunveyl work?
Alzheimer’s is characterized by dementia (problems with thinking and memory) that gets progressively worse over time.
The progression of Alzheimer’s dementia has been linked to the dysfunction and death of brain cells that are responsible for making acetylcholine, which is an important neurotransmitter (a signaling molecule that brain cells use to communicate with each other).
The severity of cholinergic loss has been found to correlate with the extent of cognitive impairment and the density of amyloid plaques, a characteristic feature observed in the brains of those with Alzheimer’s disease.
Zunveyl is a prodrug of galantamine, which means that upon entering the body, it undergoes a transformation process and is converted into galantamine. This is expected to increase its ability to be absorbed and used by the body.
As a prodrug, Zunveyl serves as a precursor to the active compound, galantamine, which is known for its therapeutic effects in treating cognitive impairment associated with Alzheimer’s.
Zunveyl is designed as a delayed-release tablet, which means it gradually releases its active ingredient over an extended period, rather than all at once. This can help maintain a consistent and steady level of the drug in the body.
Galantamine has been approved for Alzheimer’s since 2001, and was originally authorized under the brand name Razadyne. The brand-name therapy has been discontinued, but generic versions are still available.
How galantamine works to slow cognitive decline is not fully understood, but it is thought to block the activity of acetylcholinesterase, an enzyme that normally works to break down acetylcholine. By blocking this enzyme, galantamine can boost the levels of acetylcholine, which may help to normalize brain signaling.
Zunveyl also enhances the activity of two brain receptors that are sensitive to acetylcholine. This means that it modifies these receptors in a way that improves their response to the neurotransmitter.
The treatment is designed to bypass the gastrointestinal tract, thereby mitigating the common side effects associated with the oral administration of galantamine.
Who can take Zunveyl?
The U.S. Food and Drug Administration approved Zunveyl in July 2024 for adults with mild to moderate dementia of the Alzheimer’s type.
Who should not take Zunveyl?
Zunveyl should not be used by anyone with an allergy to the therapy or any of its ingredients. It is not recommended for individuals who have severe liver or kidney impairment.
How is Zunveyl administered?
Zunveyl is available as bottles of 60 delayed-release oral tablets at three dose strengths of benzgalantamine:
- 5 mg white, round, and convex, debossed with “B05” in gray
- 10 mg purple, round, and convex, debossed with “B10” in gray
- 15 mg gray, round, and convex, debossed with “B15” in dark gray
The recommended starting dose of Zunveyl is 5 mg twice daily. After at least four weeks on this starting dose, the dosage may be increased to 10 mg twice daily based on how the patient responds. After another four weeks at the 10 mg twice-daily dose, the dosage may be increased to the maximum recommended dose of 15 mg twice daily.
In patients with moderate liver impairment or moderate kidney impairment, the dosage of Zunveyl should generally not exceed 10 mg twice daily.
When treatment is interrupted for more than three days, it is recommended that patients restart Zunveyl at the lowest dosage, which should then be gradually increased to the patient’s current dose.
Zunveyl tablets should be swallowed whole and should not be split, crushed, or chewed. The therapy may be taken with or without food, but it should not be taken with alcohol. While taking Zunveyl, it is essential to maintain proper hydration by drinking enough fluids.
Zunveyl in clinical trials
FDA approval of Zunveyl was based on data from three bioequivalency studies that were conducted in healthy volunteers.
Results from each of these studies demonstrated Zunveyl was able to deliver an equivalent amount of galantamine to the body as previously approved galantamine formulations, namely immediate- and extended-release tablets. Additionally, gastrointestinal issues were observed in less than 2% of participants, and there were no reports of insomnia, two known side effects of galantamine.
Galantamine clinical studies
Previous studies of galantamine have shown it can slow the decline in standardized measures of cognitive function compared with a placebo. In those studies, Alzheimer’s patients were randomly assigned to take either immediate-release or extended-release formulations of galantamine twice-daily at various doses or a placebo, for three to six months. A three-times-daily dosing regimen was also tested but results did not suggest an advantage over twice-daily dosing.
Cognitive changes were tracked mainly using the cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-cog), which assesses different aspects of cognition such as memory, orientation, attention, reasoning, and language.
Results showed that, in patients given a placebo, average ADAS-cog scores tended to deteriorate over time, and after several months, scores were notably worse than at the start of the studies. By contrast, in patients given galantamine, scores showed stability or even slight improvement after several months of treatment, implying galantamine helped to slow the decline in cognitive function.
Scores on clinician-rated measures of cognition likewise tended to show that patients given galantamine were more likely to experience stability or slight improvement, whereas those given a placebo were more likely to worsen over time.
Real-world studies
A follow-up study of 548 patients who had participated in galantamine clinical trials suggested patients given the therapy were less likely to be placed in nursing homes.
A more recent study used data from the Swedish Dementia Registry to assess long-term outcomes with galantamine and related medications. The analysis included data on more than 10,000 patients treated with galantamine or other acetylcholine-targeting medicines, as well as more than 5,000 people who weren’t treated with such meds.
Results showed patients given acetylcholine-targeting medicines were significantly less likely to develop severe dementia or to die. For those given galantamine, the risk of severe dementia was reduced approximately 31%, and the risk of death was reduced about 29%.
Common side effects of Zunveyl
Zunveyl’s prescribing information notes common side effects of galantamine may include:
- nausea
- vomiting
- diarrhea
- dizziness
- headache
- decreased appetite.
Skin reactions
Serious skin reactions have been reported in patients treated with galantamine. Treatment with Zunveyl should be stopped immediately at the first sign of a skin rash, unless the rash is obviously not related to the therapy. If a patient shows signs of a serious skin reaction, Zunveyl should not be resumed and alternative treatments should be considered.
Anesthesia
Because of its specific mechanism of action, Zunveyl is likely to increase the effects of anesthetics of the succinylcholine type and similar medications.
Heart rhythms
Zunveyl may cause bradycardia (slowed heart rate) and abnormalities in the electrical activity of the heart. This may lead to symptoms such as syncope, which is a sudden drop in blood pressure resulting in loss of consciousness, upon rising from a seated or lying position.
Gastrointestinal complications
Zunveyl may increase the amount of acid secreted into the stomach. Patients taking the therapy, particularly those with known risk factors for stomach ulcers, should be monitored for any signs of bleeding in the digestive tract.
Because nausea, vomiting, diarrhea, and weight loss have been observed in clinical studies of galantamine, patients’ weight should be monitored when they are on Zunveyl.
Urinary problems
Acetylcholine-modulating drugs such as Zunveyl may cause difficulty in urinating, though this was not observed in trials of galantamine.
Seizures
Seizures are thought to be a potential side effect of cholinesterase inhibitors, such as Zunveyl, but they may also be a symptom of underlying Alzheimer’s. Patients should be monitored appropriately.
Lung conditions
Zunveyl should be prescribed with caution in patients who have a history of lung diseases like severe asthma or obstructive pulmonary disease. Lung function should be monitored for signs of adverse reactions.
Liver and kidney disease
Treatment with Zunveyl is not recommended for patients with severe liver or kidney problems.
Use in pregnancy and breastfeeding
There are no adequate data on the use of Zunveyl during pregnancy or while breastfeeding. Data from animal studies have suggested the therapy can cause toxic effects to a developing fetus if used during pregnancy. In these situations, the potential benefits and risks of treatment should be carefully weighed.
Alzheimer’s News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified healthcare providers with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- FDA fast-tracks expanded home injections for Alzheimer’s drug Leqembi February 2, 2026
- FDA decision on AXS-05 for Alzheimer’s agitation expected April 30 January 6, 2026
- Oral semaglutide fails to slow Alzheimer’s in pair of clinical trials December 2, 2025
- Canada gives conditional OK to early Alzheimer’s drug Leqembi November 4, 2025
- Kisunla approval offers new option to treat early Alzheimer’s in Europe October 3, 2025
Related articles