ALZ-801 Works Against Alzheimer’s by Preventing Protein Component Clumping, Study Reports
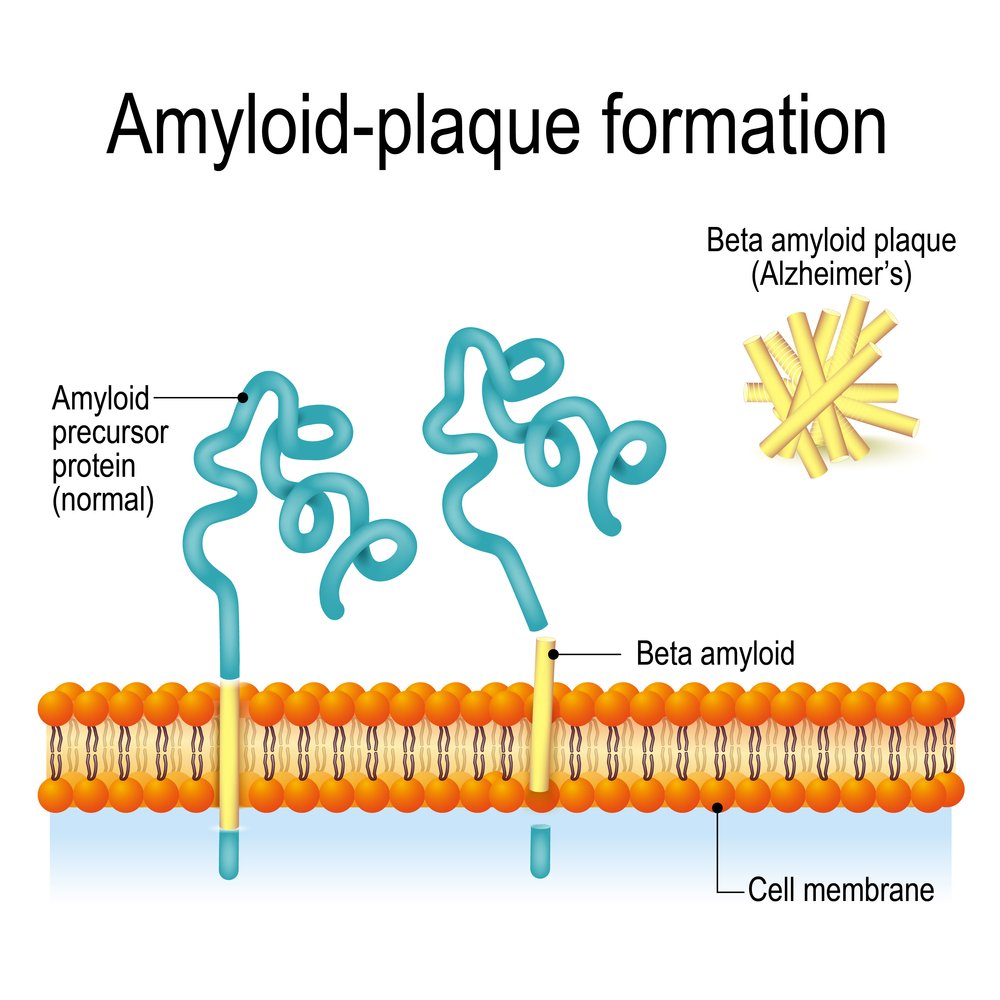
The Alzheimer’s therapy ALZ-801 works by preventing protein components from clumping together to trigger the amyloid plaque accumulation in the brain that causes the disease, researchers say.
Disease-modifying drugs for Alzheimer’s and other neurodegenerative diseases could evolve from the findings, the team said.
Researchers knew that tramiprosate, the active ingredient in Alzheon‘s ALZ-801, inhibited the beta amyloid protein that causes amyloid plaque, but they didn’t know how. Now they know.
The study, “Elucidating the Aβ42 Anti-Aggregation Mechanism of Action of Tramiprosate in Alzheimer’s Disease: Integrating Molecular Analytical Methods, Pharmacokinetic and Clinical Data,” were published in the journal CNS Drugs.
Tramiprosate is a small-molecule therapy that was evaluated in Phase 3 clinical trials for Alzheimer’s but failed to meet the study’s main effectiveness goal.
ALZ-801, which is being readied for a Phase 3 clinical trial, is an optimized version of tramiprosate. It has a better drug-property profile and better metabolic stability — or ability to remain in the body — and patients tolerate it better.
Researchers discovered that tramiprosate works by enveloping small protein components known as amyloid peptides. This prevents the peptides from clumping together to form the beta amyloid oligomers known to promote Alzheimer’s.
“While we have recognized for nearly four decades that amyloid plaques are the hallmark of Alzheimer’s disease, the emerging insights are pointing to small protein aggregates that exhibit many of the properties of prions, as the key driver of neuronal degeneration,” Dr. Stanley B. Prusiner, director of the Institute for Neurodegenerative Diseases at the University of California San Francisco, said in a press release. Prusiner, a Nobel laureate, is also chair of Alzheon’s Scientific Advisory Board.
A prion is a small particle composed of abnormally folded protein that causes neurodegenerative diseases.
“By showing the therapeutic potential to intervene in the formation of prion-like protein aggregates, this study opens the door to the development of a new class of drugs with disease-modifying potential for Alzheimer’s as well as other neurodegenerative disorders caused by protein misfolding,” Prusiner said.
After learning how tramiprosate worked, researchers wanted to find out how much of it would constitute an effective treatment. They decided to compare the amount administered with the amount of soluble amyloid beta protein in the brains of Alzheimer’s patients who participated in a previous Phase 3 study.
The amount in the patients’ brains exceeded the level needed for it to envelop the protein, the team said. This prevented all oligomer formation. The results supported the idea of ALZ-801 as an Alzheimer’s treatment.
“Previous analyses showed that tramiprosate significantly improved cognition and function in Alzheimer’s disease patients with the genetic risk factor of two ε4 alleles of apolipoprotein E (APOE4/4 homozygotes), and now we understand the mechanism supporting this clinical efficacy,” Dr. Martin Tolar, Alzheon’s president, said. “These new mechanistic data continue to strengthen Alzheon’s commitment to apply a precision medicine approach to Alzheimer’s and to confirm efficacy of ALZ-801 in a genetically defined subset of Alzheimer’s patients in our upcoming pivotal study.”