Reduced Glucose Uptake in Brain Exacerbates Alzheimer’s Disease Symptoms
 According to a recent study from researchers at the Keck School of Medicine of the University of Southern California, reduced expression of a protein called GLUT1, responsible for moving glucose in the brain-blood barrier (BBB) worsens Alzheimer’s disease cerebrovascular degeneration, neuropathology and cognitive function, suggesting that GLUT1 may represent a therapeutic target for Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. The study titled “GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration,” was published online this week in peer-reviewed scientific journal Nature Neuroscience.
According to a recent study from researchers at the Keck School of Medicine of the University of Southern California, reduced expression of a protein called GLUT1, responsible for moving glucose in the brain-blood barrier (BBB) worsens Alzheimer’s disease cerebrovascular degeneration, neuropathology and cognitive function, suggesting that GLUT1 may represent a therapeutic target for Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. The study titled “GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration,” was published online this week in peer-reviewed scientific journal Nature Neuroscience.
“Our results suggest that GLUT1 deficiencies at the blood-brain barrier are not just symptoms of Alzheimer’s but, in fact, lead to a series of vascular injuries that worsen the effects of the disease,” said Berislav V. Zlokovic, M.D., Ph.D., director of the Zilkha Neurogenetic Institute (ZNI) at the Keck School of Medicine, the Mary Hayley and Selim Zilkha Chair for Alzheimer’s Disease Research and the study’s principal investigator. “We do not know yet whether medicine can restore GLUT1 expression, but we believe that targeting the protein may help prevent Alzheimer’s from getting worse among individuals predisposed to develop the disease.”
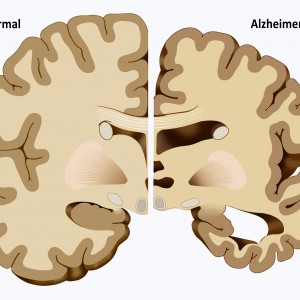
Estimates for Alzheimer’s disease show that the condition affects approximately 5.2 million people of all ages in the United States, and researchers believe that the disease will affect 16 million Americans aged over 65 years by 2050. Alzheimer’s disease causes progressive and irreversible memory, thinking and behavior impairments. GLUT1 helps glucose, the brains energy source, to move across the BBB, a layer in the brain that averts the entry of pathogens and blood into the brain.
For the study, the research team found that GLUT1 insufficiency caused reduced glucose uptake in mice over expressing amyloid β-peptide (Aβ) leading to diminished neuronal activity, behavioral deficits, and progressive neuronal loss and neurodegeneration,
Results also revealed that GLUT1 deficiency in endothelium, but not in astrocytes, initiates the vascular phenotype as shown by BBB breakdown. Alzheimer’s disease occurs due to accumulation of amyloid-beta peptide in the brain facilitated by breakdown of the BBB. Future studies could involve the identification of the metabolic pathways by which GLUT1 deficits influence metabolism and if early embryonic GLUT1 damage disturbs the central nervous system differently than a defect experienced later during development.






