Astrocytes’ Role in Neurological Disorders, Alzheimer’s Disease
Written by |
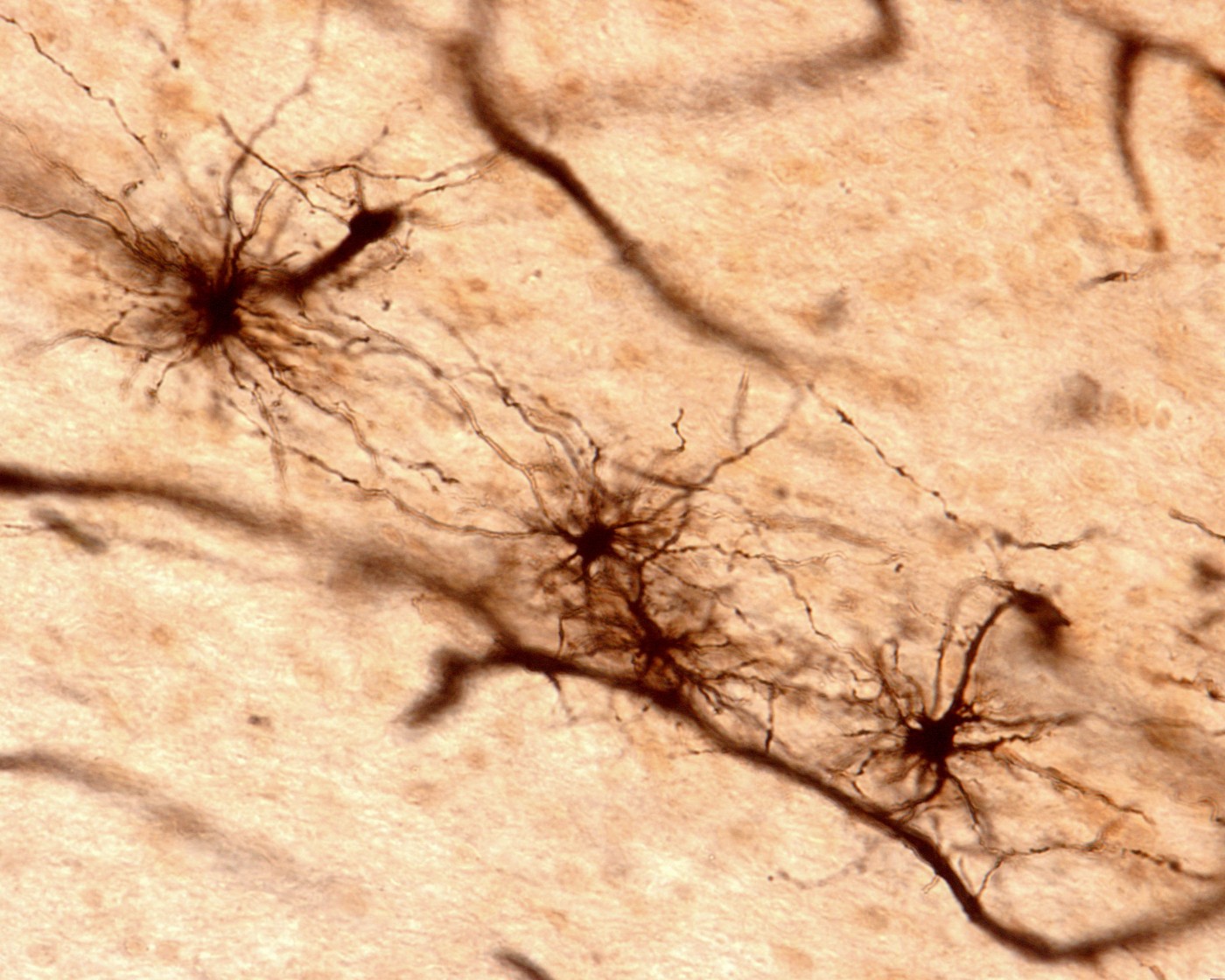
A study published in the journal Nature Neuroscience revealed new insights into the role played by astrocytes in neurological disorders such as Alzheimer’s disease. The study was conducted by an international research team and is entitled “Nuclear pore complex remodeling by p75NTR cleavage controls TGF-β signaling and astrocyte functions”.
Astrocytes are cells critical for nutrient provision to the nervous tissue, maintenance of ion homeostasis and repair mechanisms in the brain and spinal cord. Upon brain injury, astrocytes produce high amounts of p75 neurotrophin receptor (p75NTR), a protein that detects growth factors important for the stimulation of survival and differentiation in neuronal cells. One of the growth factors recognized is the transforming growth factor beta (TGF-beta), which has been found in levels higher than normal in the brain of individuals with neurological disorders.
Alzheimer’s disease is the most common form of dementia in the elderly and is characterized by cognitive and behavioral problems, and ultimately severe loss of mental function. Alzheimer’s disease is linked to the loss of neurons responsible for memory and learning. High levels of TGF-beta have been reported in Alzheimer’s disease patients and they are thought to represent a protective host response to the neuronal injury.
Researchers have discovered that the p75NTR gene plays a key role in the astrocyte–neuronal communication. The team found that treating astrocytes with TGF-beta resulted in the release of a small fragment of the p75NTR protein that was able to bind to nucleoporins, which are proteins that regulate the passage of molecules in and out of the nucleus. The binding of the p75NTR fragment to proteins in the nuclear pore complex opened up the pore and enhanced the flow into the nucleus of certain key molecules, namely Smad2 (a molecule crucial for TGF-beta to exert its effects), allowing astrocytes to enter a reactive state. In the absence of p75NTR, the transport of Smad2 into the astrocyte’s nucleus was blocked. Furthermore, p75NTR was found to be required for scar formation after neuronal injuries, and to reduce gamma oscillations in mice (patterns of neuronal activity linked to learning and memory) through the control of TGF-beta signaling.
RELATED :Alzheimer’s Disease Brain Deficits Reversed in Mouse Model By Yale School of Medicine
“Unexpectedly we may have discovered a hidden pathway to understanding how astrocytes respond to injury and control brain processes. The pathway may be common to many brain diseases and we’re just starting to follow it,” said the study’s co-senior author Dr. Katerina Akassoglou from the Gladstone Institute for Neurological Disease in a news release.
The team employed high-resolution microscopes to visualize the astrocyte nucleus in action. “Nuclear pores are gatekeepers and p75NTR appears to be the key to unlocking particular gates,” explained Dr. Akassoglou. “We discovered novel roles for both players and will continue to study how the nuclear pore complex controls neuronal development and disease.”
“This research highlights the importance of the nuclear pore complex in the brain and raises the possibility that it may be a target for treating a wide range of neurological disorders,” concluded Dr. Jill Morris, program director at the National Institute of Neurological Disorders and Stroke (NINDS).





