Alzheimer’s Disease: Zinc Transporters May Shed Light on Molecular Mechanism
Unbalanced, unhealthy zinc levels show in many diseases including Alzheimer’s disease, Parkinson’s disease, and pancreatic cancer. But lack of information about the molecular structure of zinc transporters hampers the discovery of new drugs to bring zinc to proper levels (homeostasis) with other important elements.
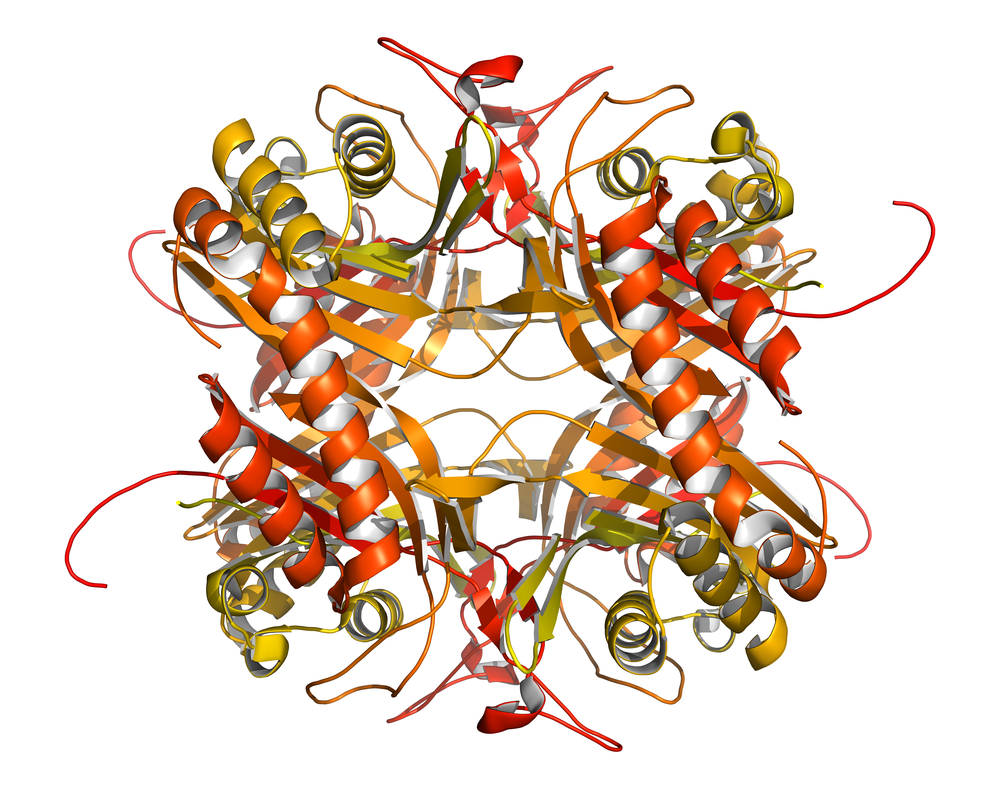
Researchers at Michigan State University (MSU) have taken a new step toward the development of such drugs, by providing a crystal structure of the extracellular domain (ECD) of ZIP4, a protein expressed by cells that line the intestine and are involved in the uptake of zinc from food.
The study, “Structural insights of ZIP4 extracellular domain critical for optimal zinc transport“, was published in Nature Communications.
Zinc ion, the second most abundant trace element in humans after iron, is essential in a variety of biological processes and must be tightly regulated. It is required for the proper function of several enzymes in the body, for the activity of proteins that regulate gene expression, and it is thought to act as a neurotransmitter.
In mammals, zinc homeostasis is mainly maintained by two zinc transporter families, the ZnT family, responsible for removing zinc from the cells, and the ZIP family which mediates zinc uptake and increases its concentration in the cell cytoplasm.
ZIP4 has been shown to be over expressed in several types of cancers, including prostate cancer. Mutations in ZIP4 lead to a rare but lethal genetic disease called ecrodermatitis enteropathica (AE), which is caused by severe zinc deficiency.
A majority of AE-causing mutations on ZIP4, that affect either its ability to transport zinc, or the protein production itself, are found in the ECD. By providing a detailed structure of the ECD, the researchers are paving the way for the development of therapies against that part of the protein and a promising strategy for regulating ZIP4 function.
“Many drug candidates fail during development because their targets are buried inside the cell,” Jian Hu, assistant professor in MSU’s chemistry department, said in a press release. “With ZIP4, though, the large ECD is fully exposed to the extracellular space and quite accessible.”
The study also shows that the ZIP4 ECD is shared among other proteins of the ZIP family, providing further insight on the structural and functional properties of other ZIP proteins known to participate in a variety of cancers and other serious diseases.
“For example, for patients suffering from diseases like Alzheimer’s or Parkinson’s, the levels of transition metals, particularly zinc and iron, in their brains are significantly higher than those of healthy people,” said Hu. “My laboratory is interested in revealing a better understanding of the body’s system of properly handling these trace elements.”






