Kisunla (donanemab-azbt) for Alzheimer’s disease
Last updated July 9, 2024, by Lindsey Shapiro, PhD

What is Kisunla for Alzheimer’s?
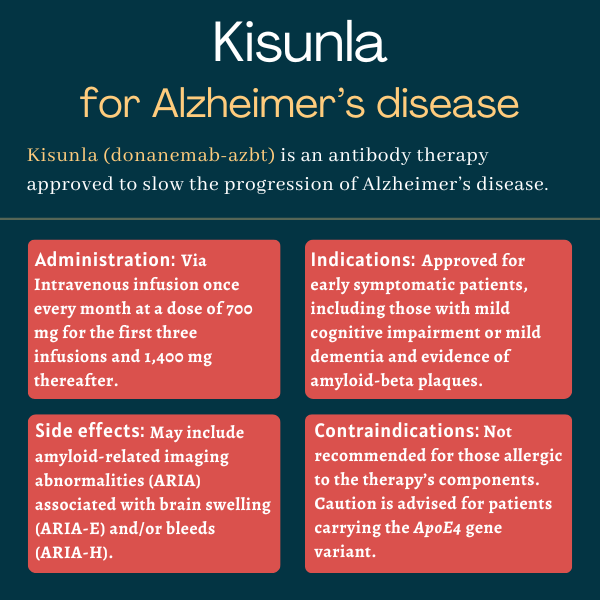
Kisunla (donanemab-azbt) is an antibody-based therapy approved in the U.S. to treat adults with early symptomatic Alzheimer’s disease. This includes people with mild cognitive impairment or mild dementia.
Developed by Eli Lilly, Kisunla is delivered via into-the-vein (intravenous) infusions and is designed to stimulate a person’s immune system to attack and destroy proteins in the brain that are believed to cause the neurodegeneration seen in Alzheimer’s. This is expected to slow the progression of the disease.
Therapy snapshot
| Brand name: | Kisunla |
| Chemical name: | Donanemab-azbt |
| Usage: | Used to treat early Alzheimer’s disease; reduces amyloid-beta plaques in the brain |
| Administration: | Intravenous infusion |
How does Kisunla work?
In Alzheimer’s disease, certain proteins, namely amyloid-beta and tau, form toxic clumps, or plaques, inside nerve cells. This is thought to contribute to the progressive dysfunction and death of nerve cells that drive the symptoms of Alzheimer’s disease.
Kisunla is an antibody designed to help clear amyloid-beta by binding to a toxic form of the protein that’s already formed into plaques and neutralizing it.
Essentially, the antibody tags the protein and marks it for clearance by the immune system. By lowering levels of amyloid-beta plaques in the brain, Kisunla is believed to be able to slow Alzheimer’s neurodegeneration and ease disease symptoms.
Who can take Kisunla?
Kisunla was approved by the U.S. Food and Drug Administration in July 2024 for adults with early symptomatic Alzheimer’s disease, which includes those with mild cognitive impairment or mild dementia and confirmed evidence of amyloid-beta plaques.
Who should not take Kisunla?
Kisunla is contraindicated, or not recommended, in those with a history of serious allergic reactions to Kisunla or any of its ingredients.
The treatment’s label has a boxed warning about the risk of amyloid-related imaging abnormalities, known as ARIA, which are classified as ARIA-E for ARIA with edema, or ARIA-H for ARIA with hemosiderin deposition. These are instances of brain swelling or brain bleeding that are common with therapies targeting amyloid-beta. In rare cases, they may be serious or life-threatening.
Patients carrying ApoE4, the strongest genetic risk factor for Alzheimer’s, may be at an increased risk for ARIA. As such, testing for ApoE4 status should be done before starting treatment.
Individuals taking medications to reduce the formation of blood clots, known as antithrombotic medicines, also may be at an increased risk of developing an ARIA.
How is Kisunla administered?
Kisunla is available as a clear to opalescent solution, colorless to slightly yellow or brown.
The treatment is supplied as a single-dose vial of 17.5 mg/mL that needs to be diluted to a final concentration of 4 to 10 mg/mL prior to administration.
Kisunla is administered by a healthcare professional as a monthly intravenous infusion, with each infusion lasting about 30 minutes. During the first three infusions, a dose of 700 mg is used, with a dose of 1,400 mg for all subsequent infusions.
If a scheduled infusion is missed, treatment should be restarted as soon as possible with the same dose given every month.
Doctors may consider discontinuing Kisunla if amyloid-beta plaques significantly decrease to minimal levels on imaging scans.
Before and during treatment with Kisunla, patients should undergo brain magnetic resonance imaging (MRI) to check for ARIA. This is particularly important during the initial six months of treatment.
If a patient experiences ARIA, dosing may need to be interrupted, depending on the severity and type of the event.
Kisunla in clinical trials
Kisunla’s approval for early Alzheimer’s was largely backed by findings from two clinical trials: the Phase 2 TRAILBLAZER-ALZ (NCT03367403) and the Phase 3 TRAILBLAZER-ALZ 2 (NCT04437511). Other trials also were conducted or are ongoing.
TRAILBLAZER-ALZ
TRAILBLAZER-ALZ enrolled 257 people with early symptomatic Alzheimer’s disease, ages 60-85, who showed signs of tau and amyloid deposits in the brain. Patients had either mild cognitive impairments, in which symptoms are present but daily life functioning is not impaired, or early dementia, where cognitive impairment is beginning to disrupt daily life.
Participants were randomly assigned to receive Kisunla (700 mg for the first three doses; 1,400 mg thereafter) or a placebo via intravenous infusion once per month for 72 weeks, or about 1.5 years. In the Kisunla group, if amyloid plaque levels declined below a certain threshold during the study, those patients were switched to the placebo.
The study’s main goal was to evaluate changes in cognitive function and related effects on daily living, as assessed by scores on the Integrated Alzheimer’s Disease Rating Scale (iADRS). Scores range from 0 to 144, with a lower score reflecting greater cognitive and functional impairments.
The results showed that patients receiving Kisunla experienced significantly slower cognitive declines than did those on the placebo, meeting the study’s main goal. Specifically, iADRS scores declined by 6.86 points over 1.5 years on Kisunla, compared with 10.06 points on the placebo. No differences were observed in most other secondary trial outcomes related to cognitive function.
Amyloid plaque levels also were significantly reduced on Kisunla relative to the placebo. By the trial’s end, about two-thirds of Kisunla-treated patients were amyloid-negative, with no notable change for those on the placebo. After about a year of treatment, more than half of Kisunla-treated patients (54.7%) exhibited large enough reductions in amyloid plaques to be switched over to the placebo.
TRAILBLAZER-ALZ 2
TRAILBLAZER-ALZ 2 was designed much like the earlier TRAILBLAZER-ALZ trial, involving a similar patient population and dosing regimen, but it enrolled substantially more patients. A total of 1,736 people were randomly assigned to receive monthly Kisunla or a placebo for about 1.5 years.
In this study, participants were divided into two groups based on tau levels in the brain — low/medium or high. The main goal was to assess changes in iADRS scores among those with low to medium tau levels and in the overall population.
That goal was met, with patients on Kisunla seeing significantly slower cognitive declines compared with those on the placebo in the low to medium tau subgroup and the overall population. Specifically, iADRS scores dropped by 6.02 points among those on Kisunla compared with a decline of 9.27 points for participants on the placebo, amounting to an approximately 35% slower cognitive decline.
Benefits from Kisunla treatment also were observed in other cognitive measures in the low/medium tau group. Moreover, fewer patients on Kisunla progressed from mild cognitive impairment to mild dementia, or from mild dementia to moderate dementia.
Among low-to-medium tau participants, Kisunla led to a nearly twofold slower cognitive decline for patients with mild cognitive impairment relative to those with early dementia. The treatment was similarly more effective in patients younger than 75 than in those 75 and older.
For the subgroup of patients with high tau levels, the benefits of Kisunla on cognitive function were not observed.
As in TRAILBLAZER-ALZ, more than half of patients on Kisunla (52%) were switched to a placebo because their amyloid levels dropped below the specified threshold.
Participants who completed the main trial could then opt to continue receiving Kisunla in an ongoing 1.5-year open-label extension portion.
TRAILBLAZER-ALZ 4
The Phase 3 TRAILBLAZER-ALZ 4 (NCT05108922) clinical trial was designed to compare the effects of Kisunla against Aduhlem (aducanemab), an anti-amyloid antibody previously sold by Biogen and Eisai, in early Alzheimer’s patients. The 148 enrolled participants were randomly assigned to receive monthly Kisunla or Aduhelm for six months.
Top-line results showed that the percentage of amyloid clearance was significantly higher with Kisunla compared with Aduhelm — 65.2% versus 17% — after six months. More than a third of patients on Kisunla (37.9%) saw decreases substantial enough that they were no longer considered amyloid-positive, compared with 1.6% of those on Aduhelm.
Ongoing studies
A number of other ongoing studies in the TRAILBLAZER clinical program are further evaluating the effects of Kisunla at different stages of Alzheimer’s disease or in different dosing regimens.
The placebo-controlled Phase 3 TRAILBLAZER-ALZ 3 trial (NCT05026866) is evaluating whether early treatment with Kisunla can prevent cognitive and functional declines in an estimated 2,600 people with preclinical Alzheimer’s disease. Among these patients, there are signs of amyloid and tau in the brain but cognitive function is still intact. The approximately 3.5-year study is expected to finish in 2027.
The TRAILBLAZER-ALZ 6 study (NCT05738486) is testing different Kisunla dosing regimens in adults with early Alzheimer’s disease. Participants will receive either of four different doses of Kisunla, interspersed with periods of placebo treatment, over six months. With a main goal of monitoring safety, the trial is expected to conclude in 2025.
Common side effects of Kisunla
The most common side effects associated with Kisunla in clinical trials include:
- amyloid-related imaging abnormalities associated with brain swelling and/or bleeds
- headache
- infusion-related reactions.
Amyloid-related imaging abnormalities
Kisunla can cause amyloid-related imaging abnormalities, or ARIA, which can be observed on MRI as either brain swelling (ARIA-E) and/or bleeding (ARIA-H).
ARIA typically occurs early in treatment and is generally asymptomatic, though rare serious events like seizures can happen. Reported symptoms may include:
- headache
- confusion
- visual changes
- dizziness
- nausea
- gait difficulty
- focal neurologic deficits.
ARIA-related symptoms often improve over time.
The risk of ARIA, including symptomatic and serious ARIA, is increased in patients carrying ApoE4, the genetic risk factor for Alzheimer’s. As such, testing for ApoE4 status should be performed before initiating treatment.
The recommendations for managing ARIA are the same for ApoE4 carriers and noncarriers.
Because ARIA with swelling mimics symptoms of stroke, healthcare providers should consider if symptoms are due to ARIA-E before administering Kisunla-treated patients medications to dissolve blood clots. Particular attention also should be given to patients who need to be on anticoagulant therapies.
Patients should carry information indicating that they are being treated with Kisunla.
Allergic reactions
Kisunla may cause hypersensitivity such as severe allergic reactions, known as anaphylaxis, and sudden, deep swelling, called angioedema. If any signs or symptoms of a hypersensitivity reaction occur, patients should immediately stop Kisunla and appropriate treatment should be administered.
Infusion-related reactions
Patients receiving Kisunla may experience infusion-related reactions. In clinical studies, these mostly occurred within the first four infusions and developed during treatment or within 30 minutes afterward.
Infusion-related reaction signs include:
- chills
- redness
- nausea
- breathing difficulty
- sweating
- high blood pressure
- headache
- chest pain
- low blood pressure.
Should one of these events occur, Kisunla infusion should be slowed or stopped, and adequate treatment should be started. Pretreatment with antihistamines, acetaminophen, or corticosteroids may help mitigate these reactions.
When considering Kisunla as a treatment option, it is important to weigh its potential benefits against the risks of serious adverse events associated with ARIA.
Use in pregnancy and breastfeeding
Insufficient data exist on Kisunla’s use in pregnant women to determine if it poses risks of birth defects, miscarriage, or other negative outcomes for mothers or their unborn babies. Additionally, no animal studies have been conducted to evaluate Kisunla’s potential impact on reproduction or development.
It also is not known if Kisunla can be found in breast milk and whether it has any impact on nursing infants. Those who plan to breastfeed should inform their healthcare team and carefully weigh the potential benefits and risks of using the medication while breastfeeding.
Alzheimer’s News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified healthcare providers with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- Researchers explore surgical treatment’s potential in Alzheimer’s March 2, 2026
- FDA fast-tracks expanded home injections for Alzheimer’s drug Leqembi February 2, 2026
- FDA decision on AXS-05 for Alzheimer’s agitation expected April 30 January 6, 2026
- Oral semaglutide fails to slow Alzheimer’s in pair of clinical trials December 2, 2025
- Canada gives conditional OK to early Alzheimer’s drug Leqembi November 4, 2025
Related articles