Ultrasound and Gas Bubbles May One Day Deliver Alzheimer’s Treatments Directly to Brain
Written by |
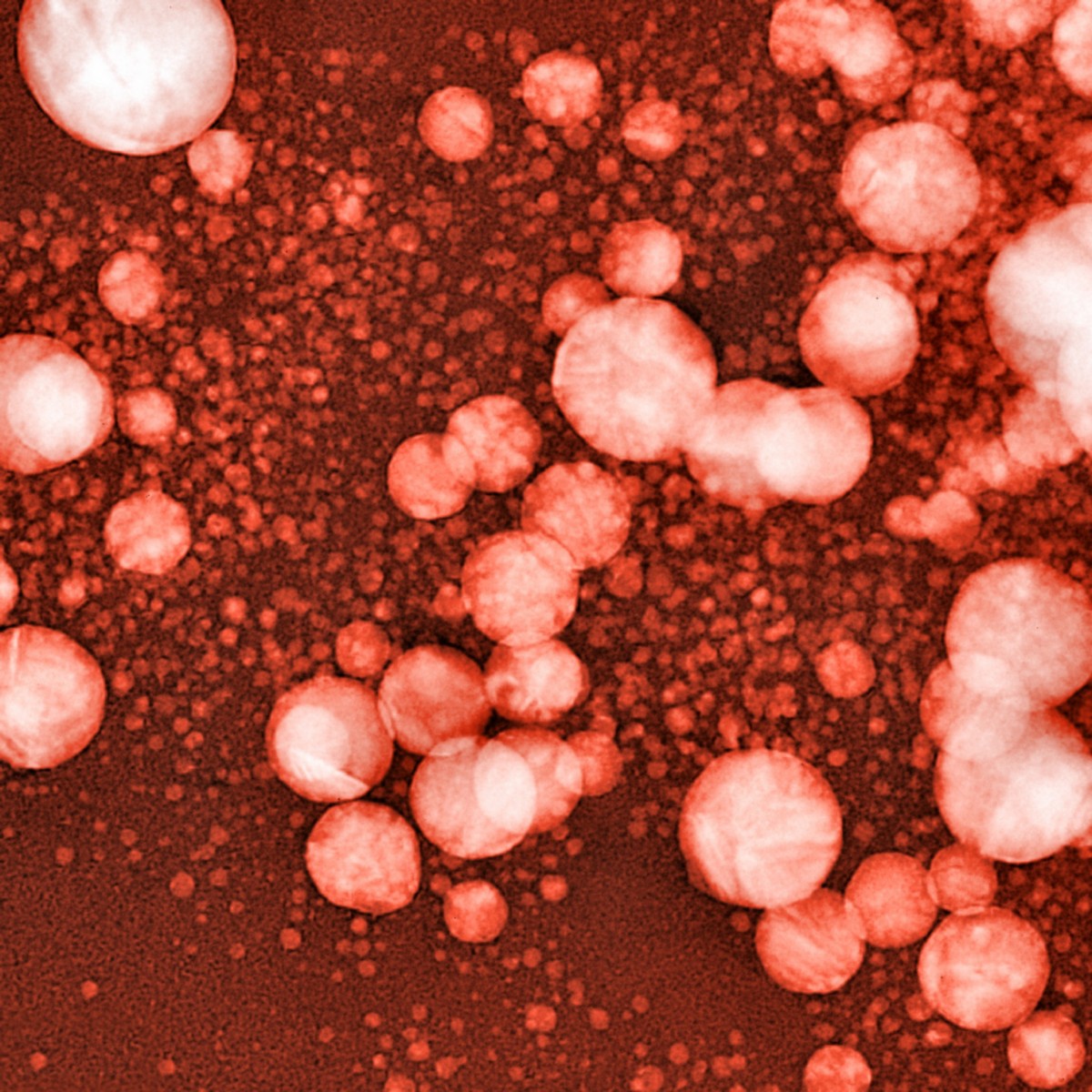
Tiny gas bubbles, embedded in a layer of fat molecules, can turn out to be the answer to a difficulty researchers have been struggling with for decades — the delivery of drugs to the brain. The method uses ultrasound to force the drug-containing bubbles over the barrier that prevents most substances from entering the brain.
Although human trials using this approach are not yet in sight, the method offers a way of manipulating drugs so that they’re likely to reach the brain of patients with Alzheimer’s disease and other brain conditions. Such treatments would have the benefit of increased efficacy with a lower risk of side effects.
The study, “Lipid microbubbles as a vehicle for targeted drug delivery using focused ultrasound-induced blood-brain barrier opening,” was published in the Journal of Cerebral Blood Flow and Metabolism.
Using ultrasound to open up the impenetrable lining of blood cells in the brain — called the blood-brain barrier — has been experimented with for years. The sound waves are known to temporarily loosen the cellular connections making up the barrier, so as to let drugs inside, but that knowledge didn’t make them an adequate drug delivery method.
When a drug that is intended for the brain circulates in the blood, it has the potential to enter other tissues — causing unwanted side effects.
Researchers at Columbia University in New York seem to have bypassed the problem. They designed tiny gas bubbles with the outer layer composed of fat molecules. When the drug is inserted into the fat layer, it stays in the bubbles as they circulated in the blood.
Then, if a beam of ultrasound is directed at a specific brain region, the sound made the bubbles oscillate and increase in size until they burst. As the sound waves in the area also open the blood-brain barrier, the drug sneaks into the brain. Soon thereafter, the barrier closes again, preventing other things from entering.
The team tested the method in mice, loading the bubbles with fluorescent molecules that allowed them to track the substance. They confirmed that the compound was unloaded in the brain, but not in other parts of the body. The study also identified parameters crucial for the work of translating this approach for human use.
“Defining these parameters means we can think about how to transfer the technique to human patients, although it has to be tested on monkeys first,” Carlos Sierra, the study’s first author, said in a news release.





