Tau Protein Leads To Neuronal Death in Alzheimer’s
Written by |
 In a study published in the online issue of Molecular Neurodegeneration journal, a team of researchers at Georgetown University Medical Center (GUMC), in collaboration with researchers at the Capital Medical University in Beijing, China and funded by Merck Research Laboratories, shows that Tau protein is the key triggering event to neuronal death in Alzheimer’s disease patients.
In a study published in the online issue of Molecular Neurodegeneration journal, a team of researchers at Georgetown University Medical Center (GUMC), in collaboration with researchers at the Capital Medical University in Beijing, China and funded by Merck Research Laboratories, shows that Tau protein is the key triggering event to neuronal death in Alzheimer’s disease patients.
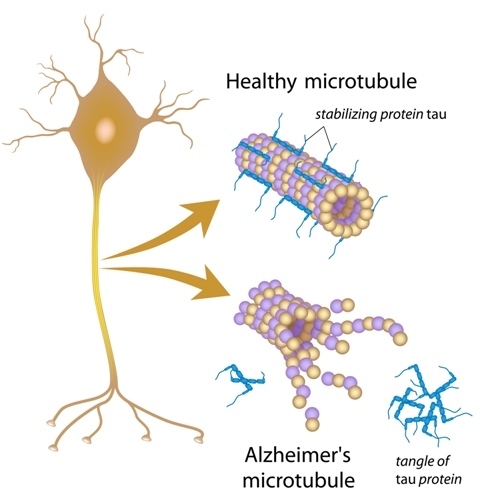
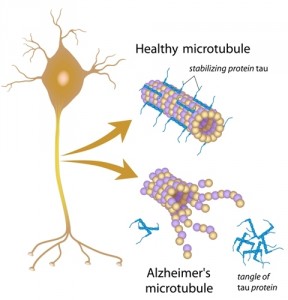
Alzheimer’s disease is characterized by both deposit of an extracellular protein, beta-amyloid (or Abeta), which leads to the formation of beta-amyloid plaques, and by abnormal function of the Tau protein. However, the prevailing theory for Alzheimer’s disease cause has been that beta-amyloid plaques lead to neural death. This protein is abundant in neural cells and is essential to stabilize axonal microtubules.
Now, this new study suggests that neuronal death is caused by Tau protein malfunction.
Charbel E-H Moussa, MB, PhD, assistant professor of neuroscience at Georgetown University Medical Center and the study’s lead author, noted, “When tau is abnormal, these proteins, which include Abeta, accumulate inside the neurons. The cells start to spit the proteins out, as best they can, into the extracellular space so that they cannot exert their toxic effects inside the cell. Because Abeta is ‘sticky,’ it clumps together into plaque.”
The authors propose that Tau protein malfunction resulting in accumulation of beta-amyloid inside the cells is the leading cause of neuron cell death. “When tau does not function, the cell cannot remove the garbage, which at that point includes Abeta as well as tangles of nonfunctioning tau, and the cell dies. The Abeta released from the dead neuron then sticks to the plaque that had been forming.”
Their experiments in animal models showed fewer plaques accumulating outside the cell in mice with fully functional Tau protein, and by expressing Tau protein in neurons that lacked the protein, there were no formations of beta-amyloid plaques.
The authors thus propose that helping neurons to prevent protein accumulation can benefit Alzheimer’s disease patients. A drug approved to treat cancer, nilotinib, was found to help neurons in this function, however it requires some functional Tau protein.
Moussa commented, “This drug can work if there is a higher percentage of good to bad tau in the cell. There are many diseases of dementia that have malfunctioning tau and no plaque accumulation, such as frontal temporal dementia linked to Parkinsonism. The common culprit is tau, so a drug that helps tau do its job may help protect against progression of these diseases.”





