Menopause, synapse health may interact to raise Alzheimer’s risk
Association stronger with earlier menopause, less so with HRT use
Written by |
Age at menopause may interact with factors related to the health of synapses, or nerve cell connections, to influence the risk of Alzheimer’s disease in women, a study found.
Associations between biomarkers of poor synapse health and higher levels of Alzheimer’s-related proteins in the brain, as well as steeper cognitive declines, were stronger when menopause was experienced at a younger age, the researchers said. These interactions were not as strong when women had received hormone replacement therapy.
“These findings highlight the importance of both hormonal factors and synaptic health in influencing AD [Alzheimer’s disease] risk in women,” Madeline Wood Alexander, the study’s first author and a graduate student at the University of Toronto and the affiliated Sunnybrook Research Institute (SRI), said in a university news story.
The study, “The interplay between age at menopause and synaptic integrity on Alzheimer’s disease risk in women,” was published in Science Advances.
Alzheimer’s is a neurodegenerative disease characterized by progressive declines in memory and other cognitive functions, ultimately leading to dementia, in which cognitive issues are severe enough to disrupt daily life.
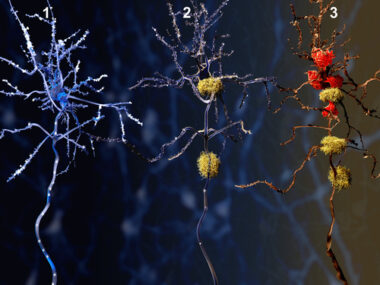
The Alzheimer’s brain is marked by the toxic accumulation of proteins, namely beta-amyloid and tau, that damage nerve cells. Synapses, the sites through which nerve cells communicate, start to be lost early in the disease course, which is strongly linked to cognitive declines.
Women see higher risk, faster disease progression
Alzheimer’s does not affect the sexes equally. Not only are women at a higher risk of the disease, but they tend to experience faster disease progression. About two-thirds of Alzheimer’s patients with dementia are women, and female patients see faster cognitive declines even when they have a similar degree of toxic proteins in the brain as their male counterparts, according to the authors.
The reasons underlying these sex disparities are not fully established, but menopause, by way of its effects on sex hormones, may play a role.
Among the changes that occur in menopause are reductions in forms of estrogen that are believed to have neuroprotective effects. Early menopause, and thus lower lifetime exposure to estrogen, has been associated with an elevated risk of dementia and a greater tau burden in the brain.
Estrogen is involved in synapse formation and function. Declines in estradiol, the most potent form of estrogen, have been found to negatively impact synapses in animal models.
“Despite the known role of estrogens in maintaining the health of the brain’s connections, there remains a notable lack of research investigating how women’s endocrine [hormonal] health factors interact with synaptic functioning to influence Alzheimer’s pathology and cognitive decline,” Wood Alexander said.
Findings from the recent study may help to fill that knowledge gap, suggesting that synaptic health and age at menopause could interact to influence Alzheimer’s risk.
The scientists looked at clinical and brain autopsy data from 268 women who had participated in the Rush Memory and Aging Project. All had experienced spontaneous menopause at a mean age of nearly 50.
Lower levels of certain synapse-related proteins — reflecting poorer synapse health — were associated with higher beta-amyloid burden, but not with tau levels or cognitive function. Age at menopause alone wasn’t associated with markers of synapse function, Alzheimer’s-related proteins, or cognitive function.
However, age at menopause was found to influence the association between synaptic biomarkers and other outcomes. Links between worse synapse health and higher tau burden were stronger with a younger age at menopause.
Likewise, younger menopause strengthened the relationship between poor synaptic health and steeper cognitive declines.
Exploratory analyses showed that these interactions were less pronounced among women who had taken hormone therapy, which helps ease menopause symptoms for some women by providing an external source of hormones such as estrogen.
Taken together, the findings suggest that synapse health and hormonal factors may interact to influence women’s Alzheimer’s vulnerability. “Interventions addressing both hormonal factors and synaptic health could enhance resilience to dementia in women,” the researchers wrote.
The mechanisms underlying these possible relationships and how they influence women’s health remain unknown. “There is a critical need for more research focused on women’s health, which has long been undervalued, understudied, and underfunded,” said Jennifer Rabin, PhD, assistant professor at the University of Toronto, SRI researcher, and a senior author on the study.
“By prioritizing research that includes women, we not only address critical gaps in knowledge but also uncover interventions that could help all brains stay healthier for longer,” Rabin said.